TEACH-FLEX
Concentrations (At Home)
Grade Levels: Middle School,High School
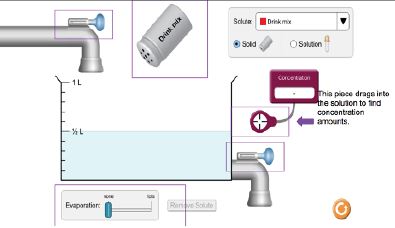
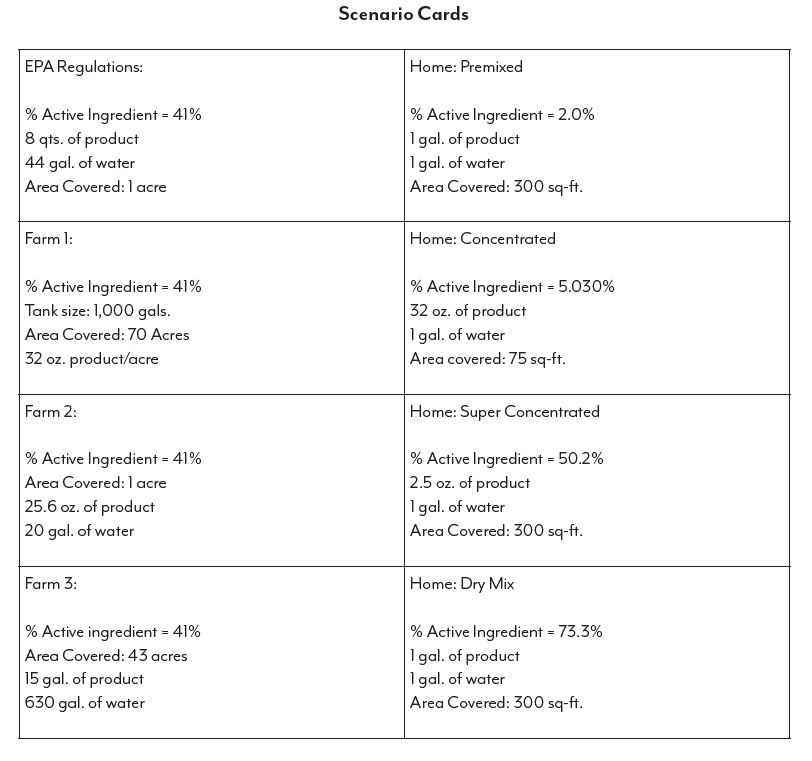
One of the biggest issues facing producers today are the misconceptions surrounding some of the most fundamental practices of production, the use of sprayed pesticides, herbicides and fertilizers. Through the ubiquitous availability of news platforms, either through social media or through traditional internet sites, the average individual is being bombarded with information that comes from a flawed understanding of the basic principles of concentrations and appropriate application. Herbicides, such as glyphosate, are readily available for purchase in formulations for home use as well as commercial agricultural formulations. This lab will investigate the non-standardized formulations of at-home glyphosate products and application suggestions as opposed to the commercial agricultural products and practices. Special attention will be paid to the ideas of concentrations, dilutions, and application surface area for both types of application situations. Is the commercial consumer really using more than the at-home consumer when we do the math? We will also consider the question: what constitutes too much application?
Teaching the Lesson
This lesson is the work product of the Kansas Corn Commission. Our lessons are written in collaboration with Kansas teachers for use in the classroom. Teachers may copy and share this curriculum. Use of this product for commercial or promotional use is prohibited without express permission of Kansas Corn.
Newsletter Sign Up
Each quarter we release a newsletter written by teachers for teachers. This is an easy way to keep up with what is happening at Kansas Corn STEM.
Subscribe Today!