Ethanol Fuel
Ethanol vs Fossil Fuel
Grade Level: Middle School
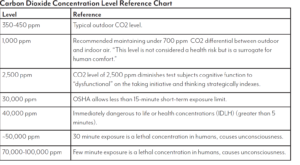
The purpose of the activities in this lab is to compare and contrast ethanol to fossil fuels. For each fuel, we will compare the amount of carbon dioxide produced, the amount of carbon soot produced and the amount of heat produced when burned.
Teaching the Lesson
Everything you need to know to teach the Ethanol vs Fossil Fuels Lesson can be found below. This lab consists of three activities to compare ethanol and kerosene. Each activity can be done independently or collectively. Each activity can be done as a whole class demonstration or as lab stations. What you do and how you do it should be determined by your learning objectives and the needs of the students in the classroom.
- Kansas College and Career Ready Standards
- Learning Objectives
- Materials
- Safety Considerations
- Procedures for Instruction
- Preparation Procedure
- Background Information
- Classroom Discussion
- Procedures for Lab
- Teacher Resources
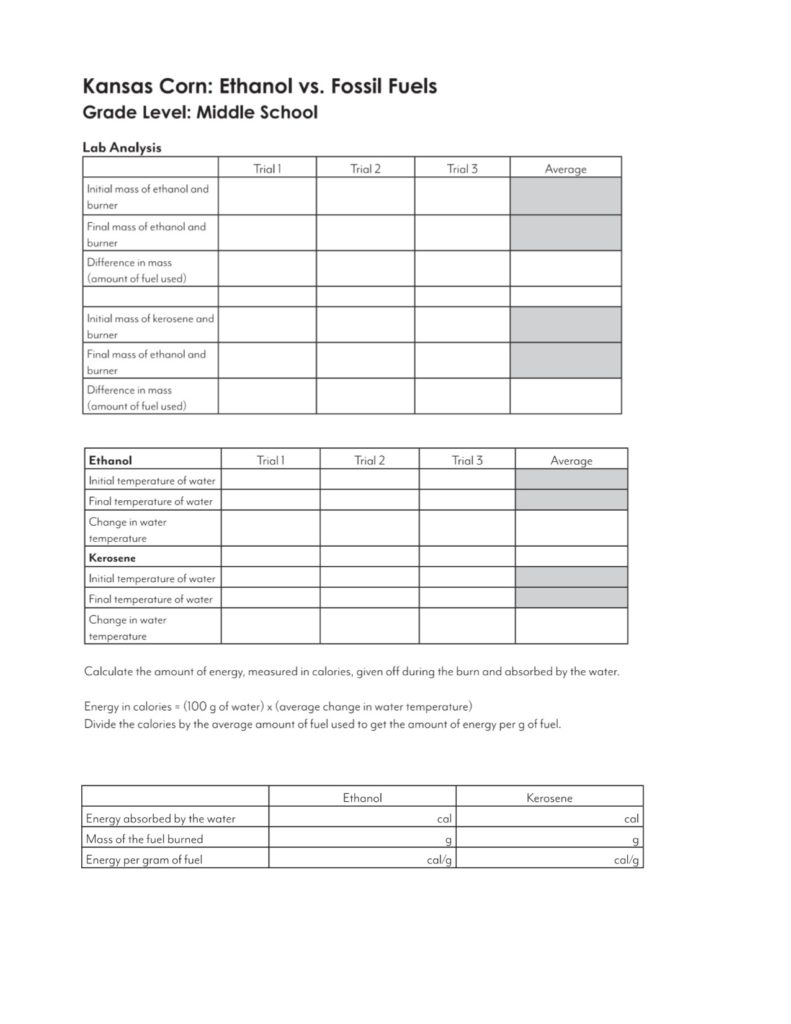
- Analysis
- Reflection and Conclusion
- Science and Agriculture Careers
- Sources
- Disclaimer
About Kansas Corn STEM
Investing in Kansas teachers and students is a priority for the Kansas Corn Commission. We are committed to providing materials and training to support STEM education while fostering an understanding of how corn farming and agriculture fit into our daily lives. Professional development workshops are offered to teachers seeking to expand their knowledge and inquiry-based teaching skills. Workshop participants receive free lab supplies needed for the lessons.
Workshop InfoThis lesson is the work product of the Kansas Corn Commission. Our lessons are written in collaboration with Kansas teachers for use in the classroom. Teachers may copy and share this curriculum. Use of this product for commercial or promotional use is prohibited without express permission of Kansas Corn.
Newsletter Sign Up
Each quarter we release a newsletter written by teachers for teachers. This is an easy way to keep up with what is happening at Kansas Corn STEM.
Subscribe Today!