Ethanol Fuel
Fermenting Fuel
Grade Level: High School
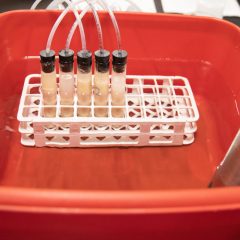
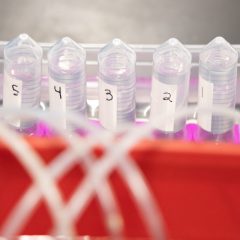
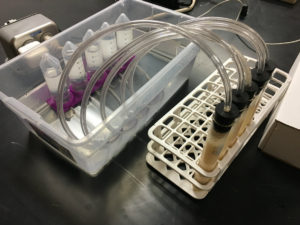
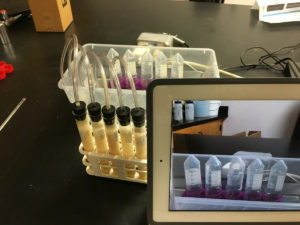
Ethanol is a renewable source of fuel for vehicles, which is widely produced from corn. Ethanol production is reliant on the anaerobic fermentation of corn sugars by yeast. Scientists and industry professionals are constantly working to make the fermentation procedure more efficient. Different enzymes are added to the corn in order to break the starch into simple sugars that the yeast can process into ethanol. This lab allows students to experiment with different variables in the fermentation process to determine their effect. Students can then use their findings to develop a fermentation procedure that they may use on a larger sample for the distillation lab, Corn Mash and Distillation.
Variables teachers and/or students can select to test:
- Feedstock: Suggest using ground corn if students are designing procedure for distillation
- Enzymes: none, alone, or in combination
- Effect of pH on yeast and enzymes
- Amount of yeast solution added
- Starting yeast in a 2% glucose solution
Students will design and conduct their own investigations to answer one or more of the following driving questions or develop their own. (Teacher may choose the questions, and it is recommended that all students test enzymes separately, mixture and control as one investigation.
- What is the effect of each enzyme or combination on glucose concentration and rate of fermentation?
- How much of an effect does starting yeast in glucose solution have on fermentation rate?
- What is the optimal amount of yeast solution per gram of feedstock to produce the most ethanol in the allotted time frame?
- Do the enzymes work more effectively at releasing glucose at a certain pH?
- How does baker’s yeast compare to brewer’s yeast in ability to produce ethanol from corn?
Students will measure the amount of CO2 produced in the time frame that the teacher selects. This can be one class period or overnight. If allowed to run overnight, time-lapse video of the fermentation allows students to plot data points from the times they were not able to directly observe the fermentation.
About Kansas Corn STEM
Investing in Kansas teachers and students is a priority for the Kansas Corn Commission. We are committed to providing materials and training to support STEM education while fostering an understanding of how corn farming and agriculture fit into our daily lives. Professional development workshops are offered to teachers seeking to expand their knowledge and inquiry-based teaching skills. Workshop participants receive free lab supplies needed for the lessons.
Workshop InfoThis lesson is the work product of the Kansas Corn Commission. Our lessons are written in collaboration with Kansas teachers for use in the classroom. Teachers may copy and share this curriculum. Use of this product for commercial or promotional use is prohibited without express permission of Kansas Corn.
Newsletter Sign Up
Each quarter we release a newsletter written by teachers for teachers. This is an easy way to keep up with what is happening at Kansas Corn STEM.
Subscribe Today!