Ethanol Fuel
More Fuel Biodiesel and Renewable Diesel
Grade Level: High School
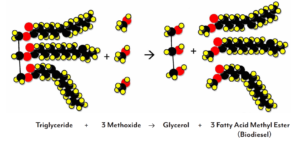
The main renewable fuel derived from corn is ethanol. Ethanol is produced when yeast ferment the sugars released from starches in the corn. In addition to starch, corn plants also store energy in the form of oil. The extraction and use of these oils as a valuable coproduct is becoming a more common practice in the production of ethanol. These oils can be used in cooking, but also in the production of fuels in the form of biodiesel or renewable diesel. Biodiesel is a renewable, clean fuel that can be mixed with conventional diesel. Biodiesel, like ethanol, is made of carbon atoms that were in the atmosphere as carbon dioxide before being fixed during photosynthesis. Biodiesel is produced when the fatty acid chains of the oil are removed in a reaction with methanol and a catalyst. Renewable diesel is also produced from different oils by a catalyst driven reaction that allows for the precise manipulation of the molecules, producing an even more stable product.
Teaching the Lesson
- Kansas College and Career Ready Standards
- Learning Objectives
- Materials
- Safety Considerations
- Procedure for Instructions
- Preparation Procedure
- Background Information
- Classroom Discussion
- Procedures for Lab
- Teacher Resources
- Lab Analysis
- Reflection and Conclusion
- Science and Agriculture Careers
- Sources
- Disclaimer
This lesson is the work product of the Kansas Corn Commission. Our lessons are written in collaboration with Kansas teachers for use in the classroom. Teachers may copy and share this curriculum. Use of this product for commercial or promotional use is prohibited without express permission of Kansas Corn.
Newsletter Sign Up
Each quarter we release a newsletter written by teachers for teachers. This is an easy way to keep up with what is happening at Kansas Corn STEM.
Subscribe Today!