Explore Corn
How Sweet It Is
Grade Level: High School
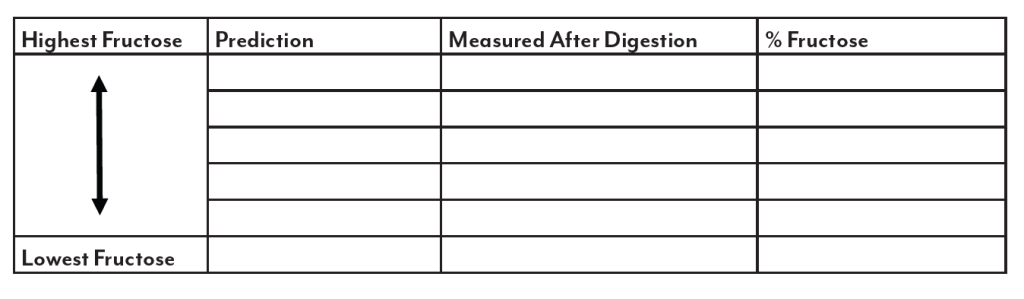
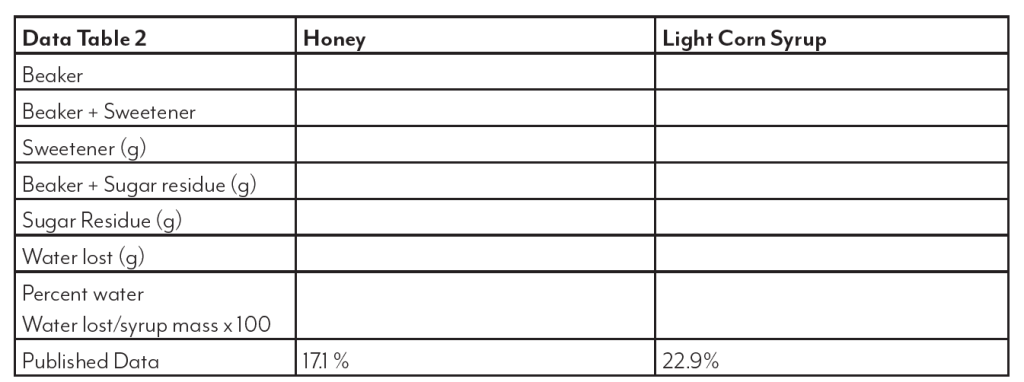
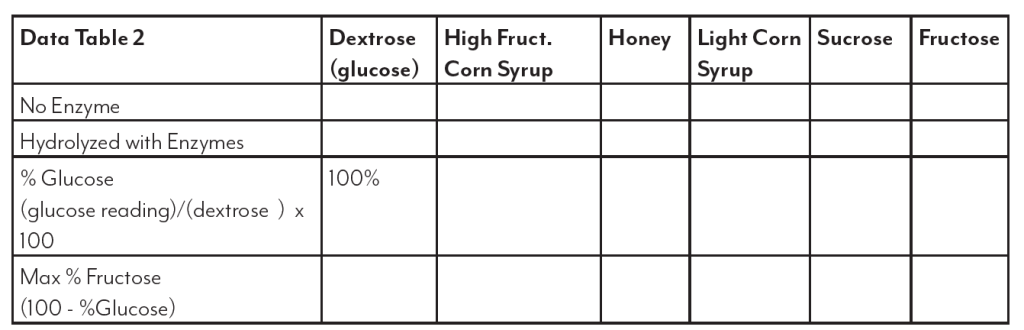
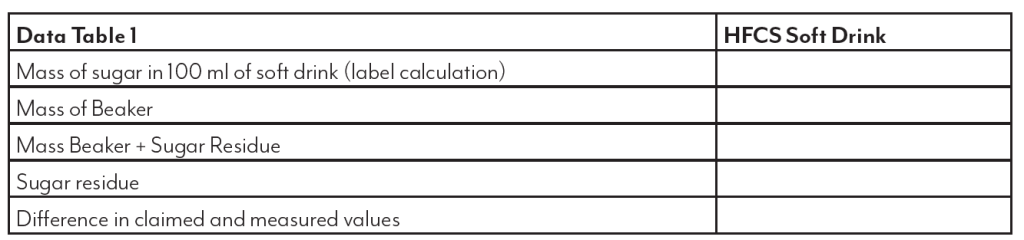
High fructose corn syrup (HFCS) is a sweetener produced from corn starch. This sweetener is used in many applications and has received much criticism and blame for health problems such as obesity and diabetes. This is attributed to the “high” fructose content indicated in the name. While the use of this sweetener is important to the corn industry, it is equally important to examine any truth to the claims. This lab will look at the sugar make up of HFCS as found in bottled soft drinks and compare it to table sugar (sucrose), dextrose (glucose), light corn
syrup and the “natural” sweetener, honey.
Teaching the Lesson
About Kansas Corn STEM
Investing in Kansas teachers and students is a priority for the Kansas Corn Commission. We are committed to providing materials and training to support STEM education while fostering an understanding of how corn farming and agriculture fit into our daily lives. Professional development workshops are offered to teachers seeking to expand their knowledge and inquiry-based teaching skills. Workshop participants receive free lab supplies needed for the lessons.
Workshop InfoThis lesson is the work product of the Kansas Corn Commission. Our lessons are written in collaboration with Kansas teachers for use in the classroom. Teachers may copy and share this curriculum. Use of this product for commercial or promotional use is prohibited without express permission of Kansas Corn.
Newsletter Sign Up
Each quarter we release a newsletter written by teachers for teachers. This is an easy way to keep up with what is happening at Kansas Corn STEM.
Subscribe Today!