Ethanol Fuel
Top Fuel
Grade Level: High School
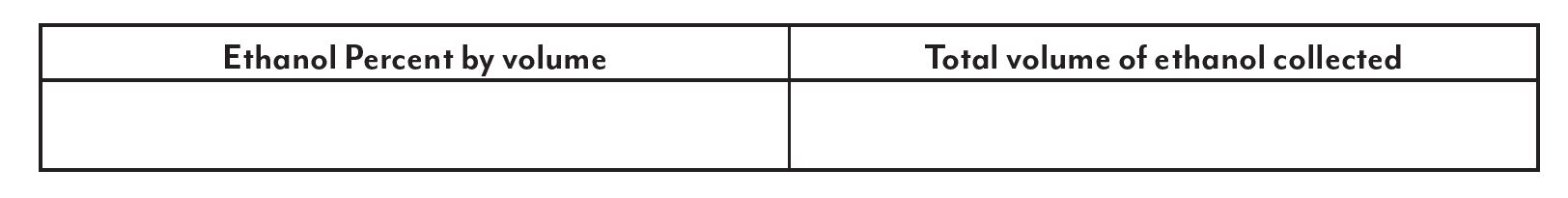
In this lab, students will learn about ethanol and its important role in our world’s ever-increasing demand for energy. After students go through the process of fermenting and distilling corn for ethanol production this lab allows them to analyze and compare their fuel. It is suggested that this lab be used as a follow-up after performing Fermenting Fuel – Designing a Procedure for Fast Fermentation and Corn Mash and Distillation. When using this approach to the lab, students use their data to produce their own procedure and compete to see which group can produce the most efficient fermentation and distill the highest volume and percentage of ethanol. This lab could also be used following a distillation of a mixture of ethanol and water.
This lesson is the work product of the Kansas Corn Commission. Our lessons are written in collaboration with Kansas teachers for use in the classroom. Teachers may copy and share this curriculum. Use of this product for commercial or promotional use is prohibited without express permission of Kansas Corn.
Newsletter Sign Up
Each quarter we release a newsletter written by teachers for teachers. This is an easy way to keep up with what is happening at Kansas Corn STEM.
Subscribe Today!